
As the luminescence did not depend on what gas had been in the vacuum or what metal the electrodes were made of, he surmised that the rays were a property of the electric current itself. English physicist and chemist William Crookes investigated cathode rays in 1879 and found that they were bent by a magnetic field the direction of deflection suggested that they were negatively charged particles. The shadow proved that the cathode rays originated from the cathode. Hittorf saw a shadow cast by an object placed in front of the cathode.
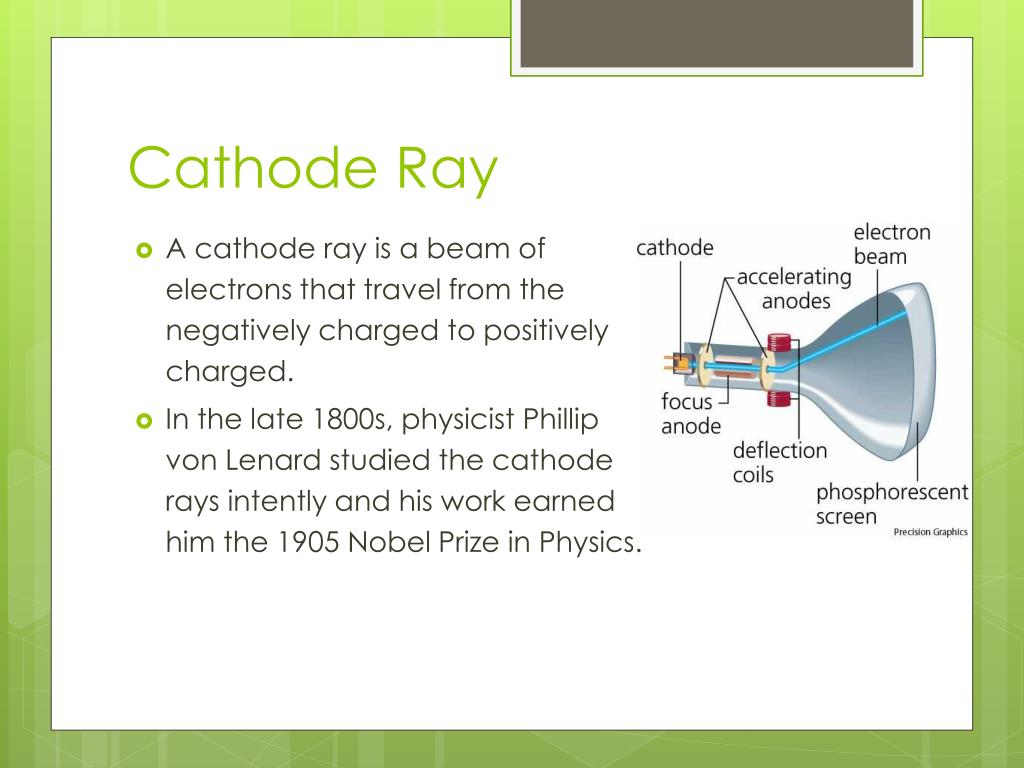
In 1869, with better vacuums, Plücker’s pupil Johann W. He found a green glow on the wall of his glass tube and attributed it to rays emanating from the cathode. Plücker discovered cathode rays in 1858 by sealing two electrodes inside the tube, evacuating the air, and forcing electric current between the electrodes. The existence of the electron showed that the 2,000-year-old conception of the atom as a homogeneous particle was wrong and that in fact the atom has a complex structure.Ĭathode-ray studies began in 1854 when Heinrich Geissler, a glassblower and technical assistant to German physicist Julius Plücker, improved the vacuum tube. Their work culminated in the discovery by English physicist J.J.
#Cathode ray experiment significance how to

Britannica Explains In these videos, Britannica explains a variety of topics and answers frequently asked questions.Britannica Classics Check out these retro videos from Encyclopedia Britannica’s archives.


 0 kommentar(er)
0 kommentar(er)
